Recent research shows that ammonia can become a promising fuel candidate for making long-haul shipping green. Ammonia does not emit CO2 and has otherwise low emissions of pollutants. Extensive use in agriculture ensures a worldwide infrastructure already in place.
Traditionally, these ships’ internal combustion engines have run on the cheapest fossil fuels. Typically bunker oil, which produces large emissions of both climate and air-polluting gases.

The shipping industry wants climate-friendly solutions, but since technology based on batteries or fuel cells is neither technically mature nor competitive in price for long distances, this is not presently an option for the shipping industry.
Modified combustion engines
A climate-friendly solution introduced in the shipping industry is to modify traditional internal combustion engines to burn carbon-free fuels to replace fossil fuels. This means engines that can run on both ammonia, natural gas, or on conventional spare fuel such as diesel when needed. However, significant research efforts are required to enable the same engine to burn different types of fuel while providing high efficiency, safety, and low emissions.
Optimisation of combustion also requires a knowledge-based approach and a detailed understanding of the basic properties of the physical processes that happen in the internal combustion engines. This is what Andrea Gruber and fellow researchers at SINTEF and NTNU are investigating in more detail now.
Unparalleled computational resources
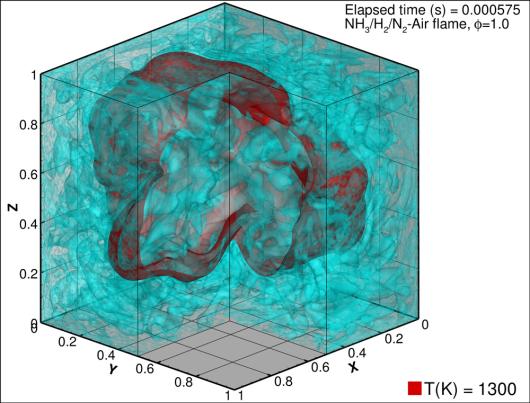
The team of researchers has conducted direct numerical simulation (DNS) to study processes in the internal combustion engine, utilising Norway's most powerful and latest HPC system Betzy. They did this by using a conventional gas fuel such as gaseous methane (CH4), carbon-free hydrogen (H2), or a mixture of ammonia and hydrogen (NH3 / H2).
DNS usually runs as massively parallel calculations using thousands of arithmetic nodes and tens of thousands of processor cores. Carrying out the simulations, the researchers have used computational resources of an order of magnitude unparalleled in a Norwegian context. With a regular laptop computer, it would take 1,168 years to perform the simulations. On Betzy, such a job takes 10 days if you use the whole machine.
Utilising waste heat to decompose ammonia on board
The calculations make it possible to simulate virtual flames in a small but representative part of a combustion chamber. Research shows that under normal operating conditions, hydrogen has too high a reactivity as a fuel and a tendency to ignite prematurely, while ammonia ignites and burns poorly. None of the fuels is therefore favourable for the internal combustion engines. However, by utilizing waste heat from the ship's engine, ammonia can be partially decomposed on board. Then we get a fuel mixture of ammonia, hydrogen, and nitrogen. A process like this is convenient for two reasons.

Firstly, the engine's waste heat is recycled to increase the energy content of the fuel. This means that the overall efficiency is improved. Secondly, the combustion properties of the new NH3 / H2 / N2 fuel mixture (ammonia/hydrogen/nitrogen) will be alike what can be characterised as standard natural gas. This is important because it entails that the engines in the current fleet should be able to combust the fuel mixture.
A comprehensive and detailed analysis of the large DNS datasets from the calculations on Betzy is underway. Strategies are specifically considered to reduce the formation of air pollutants (NOx) and greenhouse gases (N2O) from the combustion process.